Understanding Periodic Trends: A Comprehensive Guide for Students
Understanding Periodic Trends: A Comprehensive Guide for Students
Introduction
In this auspicious occasion, we are delighted to delve into the intriguing topic related to Understanding Periodic Trends: A Comprehensive Guide for Students. Let’s weave interesting information and offer fresh perspectives to the readers.
Table of Content
Understanding Periodic Trends: A Comprehensive Guide for Students
/chart-of-periodic-table-trends-608792-v1-6ee35b80170349e8ab67865a2fdfaceb.png)
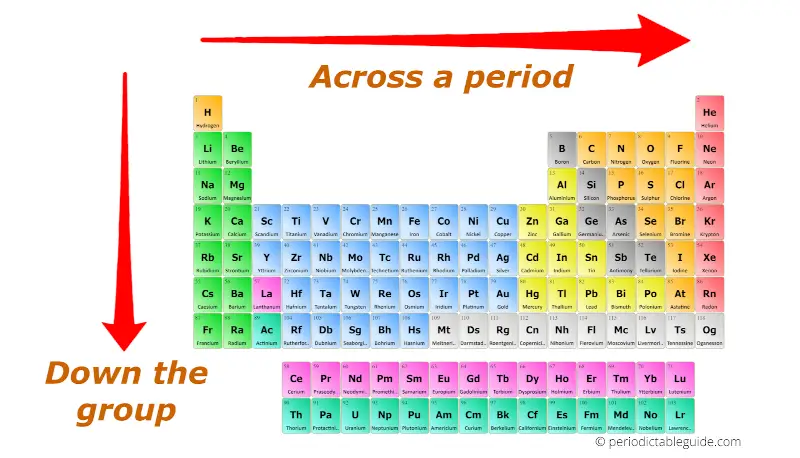
The periodic table is a cornerstone of chemistry, organizing elements based on their atomic structure and recurring properties. Understanding periodic trends – the systematic changes in element properties as you move across a period (row) or down a group (column) – is crucial for comprehending chemical behavior.
Periodic trends worksheet answers key 2025 serves as a valuable tool for students to solidify their understanding of these trends. This guide will explore the key periodic trends, their underlying principles, and how they can be applied to predict and explain chemical behavior.
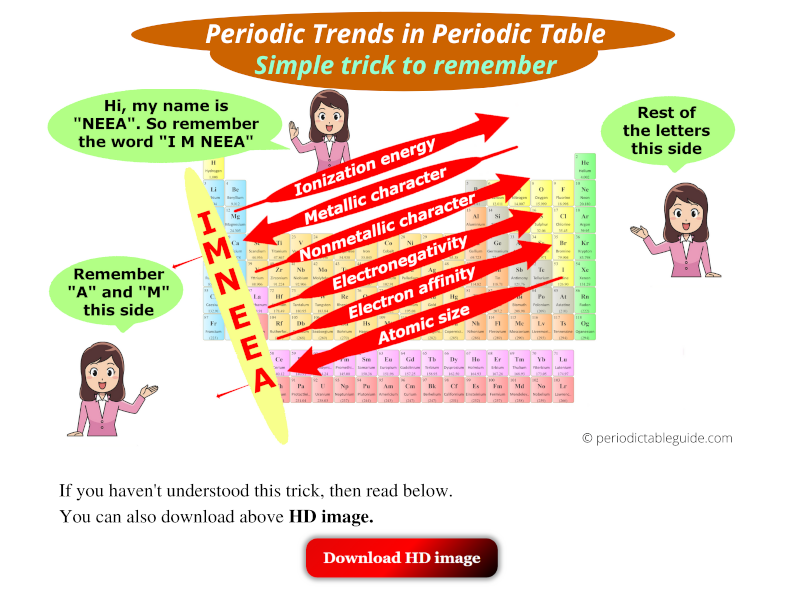
Key Periodic Trends
The periodic table exhibits several key trends:
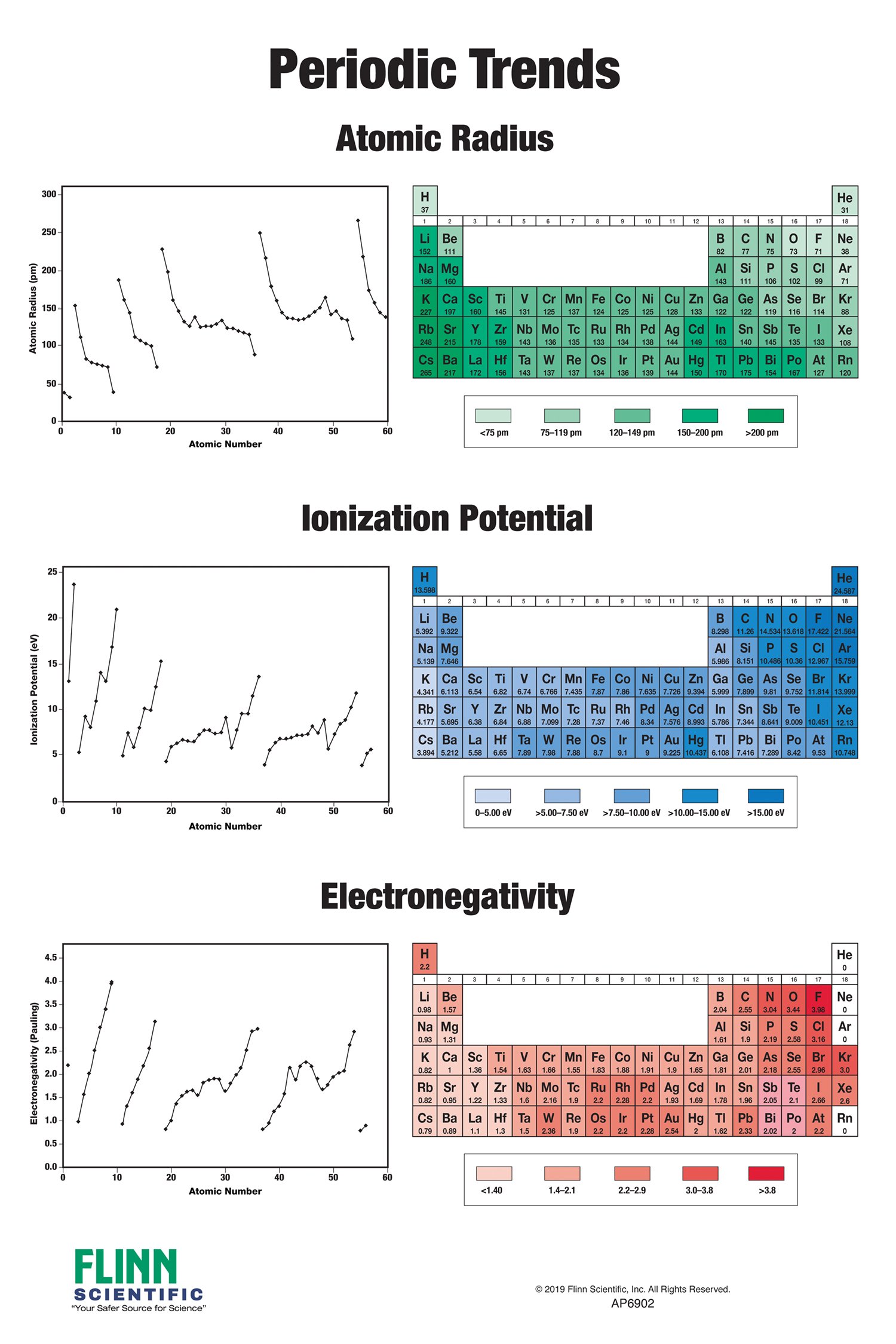
- Atomic Radius: The size of an atom, defined as the distance from the nucleus to the outermost electron shell.
- Ionization Energy: The minimum energy required to remove an electron from a gaseous atom in its ground state.
- Electron Affinity: The change in energy when an electron is added to a neutral atom in its gaseous state to form a negative ion.
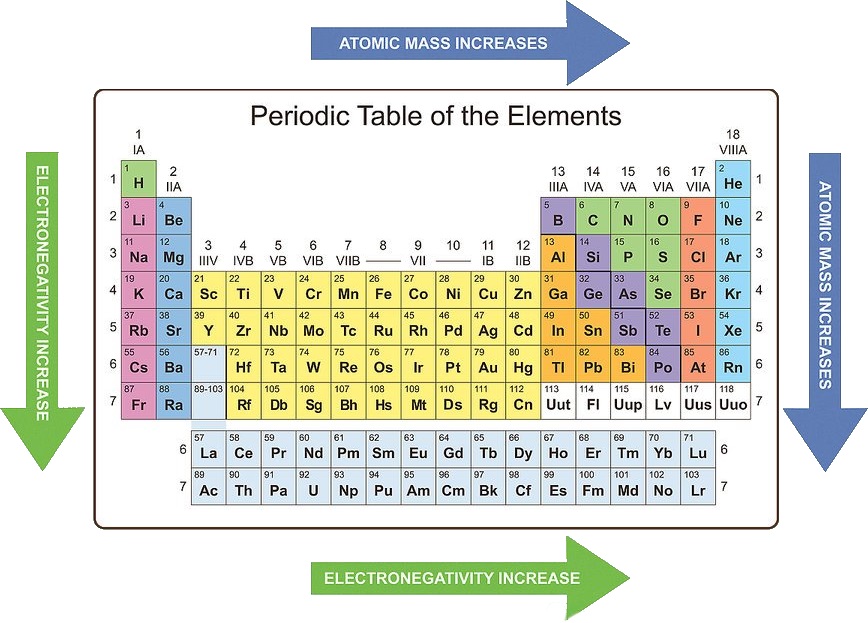
- Electronegativity: The ability of an atom to attract electrons in a chemical bond.
- Metallic Character: The tendency of an element to lose electrons and form positive ions (cations).
- Non-Metallic Character: The tendency of an element to gain electrons and form negative ions (anions).
Understanding the Trends
These trends are not arbitrary; they are rooted in the fundamental structure of atoms.
-
Atomic Radius: As you move across a period, atomic radius decreases due to increasing nuclear charge attracting electrons more strongly, pulling the electron shells closer to the nucleus. Conversely, as you move down a group, atomic radius increases because the number of electron shells increases, leading to a greater distance between the nucleus and the outermost electrons.
-
Ionization Energy: Ionization energy generally increases across a period because the increasing nuclear charge holds electrons more tightly. Down a group, ionization energy decreases because the outermost electrons are further from the nucleus and experience weaker attraction, making them easier to remove.
-
Electron Affinity: Electron affinity generally increases across a period due to the increasing nuclear charge attracting incoming electrons more effectively. However, it can decrease down a group as the outermost electrons are further from the nucleus and experience weaker attraction.
-
Electronegativity: Electronegativity increases across a period due to the increasing nuclear charge attracting electrons more strongly. It decreases down a group because the outermost electrons are further from the nucleus and experience weaker attraction.
-
Metallic Character: Metallic character increases down a group due to the decreasing ionization energy, making it easier for atoms to lose electrons. It decreases across a period due to the increasing ionization energy, making it harder for atoms to lose electrons.
-
Non-Metallic Character: Non-metallic character increases across a period due to the increasing electronegativity, making it easier for atoms to gain electrons. It decreases down a group due to the decreasing electronegativity, making it harder for atoms to gain electrons.
Applying Periodic Trends
Understanding periodic trends allows us to:
- Predict Chemical Properties: By knowing the position of an element in the periodic table, we can predict its reactivity, bonding behavior, and other chemical properties.
- Explain Chemical Reactions: Periodic trends help explain why certain reactions occur and others do not. For instance, understanding electronegativity differences can predict the type of bond formed between atoms.
- Design New Materials: The knowledge of periodic trends is crucial in material science to design materials with specific properties.
Using Periodic Trends Worksheet Answers Key 2025
- Practice and Reinforcement: The worksheet provides opportunities for students to apply their knowledge of periodic trends through various exercises.
- Identify Gaps in Understanding: By reviewing the answers, students can identify areas where they need further study or clarification.
- Develop Problem-Solving Skills: The worksheet encourages students to think critically and apply their understanding of periodic trends to solve problems.
Related Searches
1. Periodic Trends Worksheet Answers Key 2025 PDF: Numerous websites and online resources provide printable PDF versions of periodic trends worksheets with answer keys. These PDFs are useful for students who prefer working offline or for teachers who want to distribute the worksheets to their classes.
2. Periodic Trends Worksheet High School: Worksheets specifically designed for high school chemistry students cover a wider range of topics, including advanced concepts like ionization energy trends and electronegativity calculations.
3. Periodic Trends Worksheet Answers Key College: College-level worksheets delve deeper into the theoretical underpinnings of periodic trends and often include more complex problems involving atomic structure and quantum mechanics.
4. Periodic Trends Worksheet Answers Key Chemistry: These worksheets focus on the fundamental principles of periodic trends, providing a solid foundation for understanding chemical behavior.
5. Periodic Trends Worksheet Answers Key AP Chemistry: Worksheets designed for Advanced Placement (AP) Chemistry students are particularly challenging and often include questions that require in-depth analysis and application of multiple periodic trends.
6. Periodic Trends Worksheet Answers Key GCSE: GCSE (General Certificate of Secondary Education) level worksheets are suitable for students preparing for standardized exams in the UK. These worksheets typically cover basic periodic trends and their applications in chemical reactions.
7. Periodic Trends Worksheet Answers Key IB Chemistry: IB (International Baccalaureate) Chemistry worksheets are designed to meet the demanding standards of the IB curriculum. They often include challenging questions that require a thorough understanding of periodic trends and their applications in various contexts.
8. Periodic Trends Worksheet Answers Key A Level Chemistry: A Level Chemistry worksheets are designed for students in the UK preparing for advanced-level exams. These worksheets cover a wide range of topics, including advanced periodic trends and their applications in organic and inorganic chemistry.
FAQs
Q: What are the most important periodic trends to focus on?
A: The most important periodic trends to focus on are atomic radius, ionization energy, electronegativity, and metallic character. These trends form the basis for understanding chemical bonding, reactivity, and other key chemical properties.
Q: How can I use periodic trends to predict the reactivity of an element?
A: Elements with low ionization energies tend to be highly reactive metals because they easily lose electrons. Elements with high electronegativity tend to be highly reactive nonmetals because they readily gain electrons.
Q: What are some examples of how periodic trends are used in real life?
A: Periodic trends are used in various fields, including:
- Material Science: To design materials with specific properties, such as strength, conductivity, and heat resistance.
- Medicine: To develop new drugs and therapies by understanding the interactions between molecules and their target sites.
- Environmental Science: To study the impact of pollutants on the environment and develop solutions to mitigate their effects.
Tips
- Visualize the Periodic Table: Use a periodic table as a visual aid to understand the trends.
- Practice, Practice, Practice: Work through various periodic trends worksheets and problems to solidify your understanding.
- Connect Trends to Concepts: Relate periodic trends to other concepts in chemistry, such as atomic structure and bonding.
- Seek Help When Needed: Don’t hesitate to ask your teacher or tutor for clarification if you encounter difficulties.
Conclusion
Periodic trends worksheet answers key 2025 provides a valuable resource for students to master the fundamentals of periodic trends. By understanding these trends, students can develop a deeper appreciation for the organization and behavior of elements, paving the way for a comprehensive understanding of chemistry. As students progress in their studies, they will find that periodic trends serve as a powerful tool for predicting chemical properties, explaining chemical reactions, and designing new materials.
/periodictrendstable-5c4a46614cedfd000187c5db.jpg)
.PNG)


Closure
Thus, we hope this article has provided valuable insights into Understanding Periodic Trends: A Comprehensive Guide for Students. We appreciate your attention to our article. See you in our next article!