Understanding Periodic Trends: A Comprehensive Guide
Understanding Periodic Trends: A Comprehensive Guide
Introduction
In this auspicious occasion, we are delighted to delve into the intriguing topic related to Understanding Periodic Trends: A Comprehensive Guide. Let’s weave interesting information and offer fresh perspectives to the readers.
Table of Content
Understanding Periodic Trends: A Comprehensive Guide
/chart-of-periodic-table-trends-608792-v1-6ee35b80170349e8ab67865a2fdfaceb.png)
The periodic table, a fundamental tool in chemistry, organizes elements based on their atomic structure and recurring chemical properties. This arrangement reveals fascinating patterns known as periodic trends, which describe how the properties of elements change systematically across the table. Understanding these trends is crucial for predicting and explaining chemical behavior.
Exploring Periodic Trends:
-
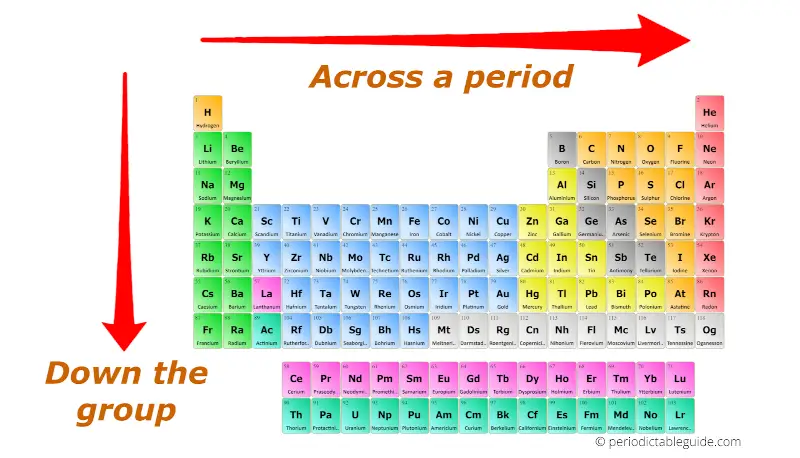
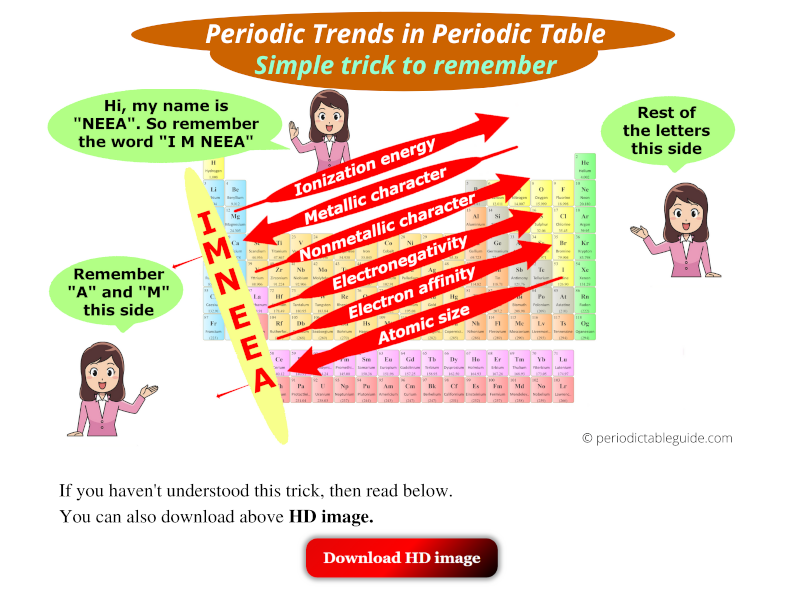
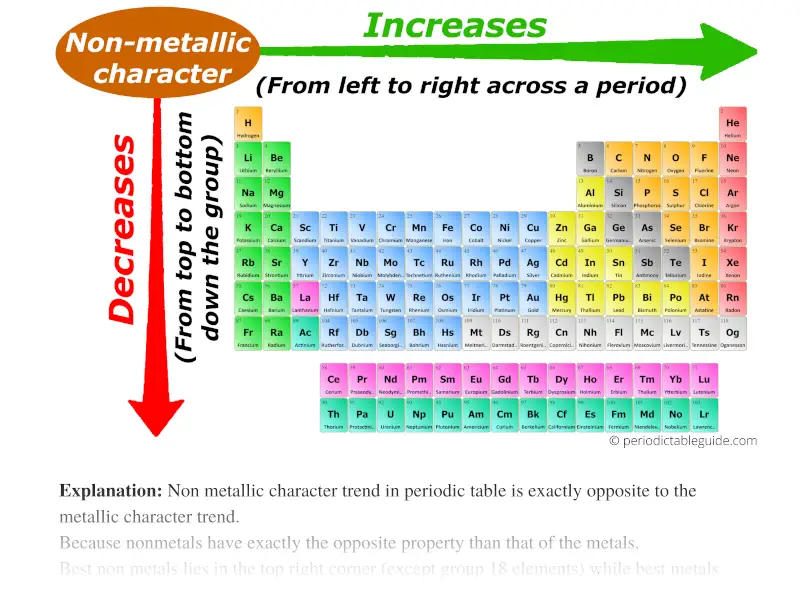
Atomic Radius: The atomic radius refers to the distance between the nucleus and the outermost electron shell of an atom. As you move down a group (column) of the periodic table, the atomic radius increases due to the addition of electron shells. Conversely, moving across a period (row) from left to right, the atomic radius decreases because the increasing nuclear charge pulls the electrons closer to the nucleus.
-
Ionization Energy: Ionization energy represents the minimum energy required to remove an electron from a gaseous atom. As you move down a group, ionization energy decreases because the outermost electron is further from the nucleus and experiences weaker attraction. Moving across a period, ionization energy generally increases due to the stronger attraction between the nucleus and the outermost electron.
-
Electron Affinity: Electron affinity measures the change in energy when an electron is added to a neutral atom in the gaseous state. Generally, electron affinity increases across a period as the nucleus becomes more effective at attracting an additional electron. However, there are exceptions to this trend. Moving down a group, electron affinity generally decreases due to the increased distance between the nucleus and the incoming electron.
-
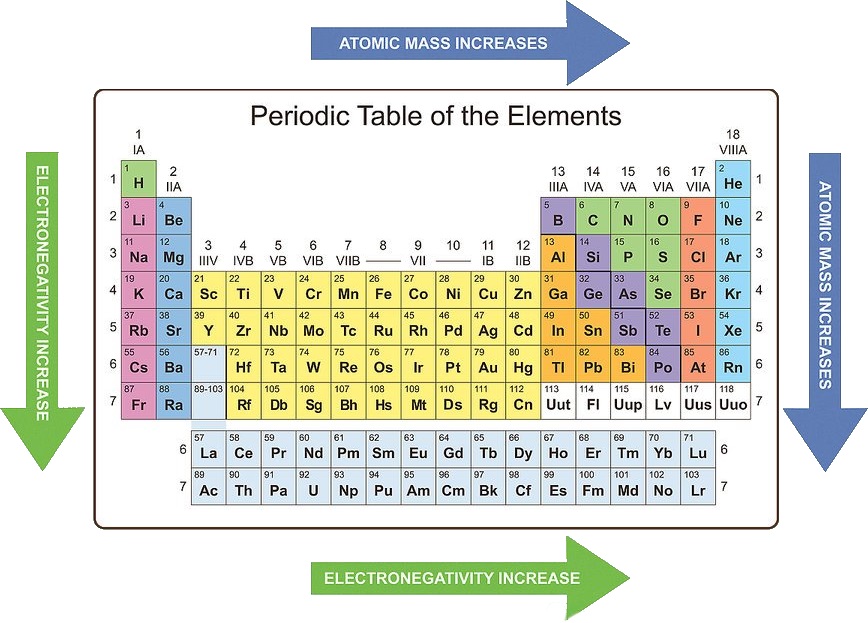
Electronegativity: Electronegativity quantifies an atom’s ability to attract electrons in a chemical bond. It generally increases across a period due to the increasing nuclear charge. Moving down a group, electronegativity decreases as the outermost electron is further from the nucleus and experiences weaker attraction.
The Importance of Periodic Trends:
Understanding periodic trends is paramount for several reasons:
-
Predicting Chemical Properties: Periodic trends enable chemists to predict the reactivity and bonding behavior of elements. For instance, knowing the electronegativity of elements allows us to predict the type of bond (ionic or covalent) they will form.
-
Explaining Chemical Reactions: Periodic trends provide a framework for explaining why certain reactions occur and others do not. For example, the tendency of alkali metals (Group 1) to lose electrons readily explains their high reactivity with water.
-
Designing New Materials: By understanding the relationship between atomic structure and properties, scientists can design new materials with specific characteristics. For example, the knowledge of ionization energy helps in developing materials with desired electrical conductivity.
Applications of Periodic Trends:
-
Chemistry Education: Periodic trends form the foundation of introductory chemistry courses, providing students with a fundamental understanding of chemical behavior.
-
Industrial Processes: Chemical engineers leverage periodic trends to optimize industrial processes, such as the production of fertilizers, plastics, and pharmaceuticals.
-
Material Science: Scientists utilize periodic trends to design and synthesize new materials with specific properties, including high-strength alloys, efficient catalysts, and advanced semiconductors.
Frequently Asked Questions (FAQs):
-
What are the exceptions to periodic trends?
While periodic trends generally hold true, there are exceptions due to factors like electron configurations, shielding effects, and the presence of filled or half-filled subshells. For example, the ionization energy of nitrogen is higher than that of oxygen, despite being in the same period, due to the stable half-filled p-orbital configuration in nitrogen.
-
How can I remember periodic trends?
There are various mnemonics and visual aids to help remember periodic trends. One popular mnemonic is "All Students Take Chemistry Exams For Increased Numbers" to remember the trends in atomic radius, ionization energy, and electronegativity across a period.
-
Can periodic trends be used to predict the behavior of unknown elements?
Yes, periodic trends can be used to predict the properties of unknown elements. By extrapolating the trends observed for known elements, scientists can make educated guesses about the behavior of elements yet to be discovered.
Tips for Mastering Periodic Trends:
-
Visualize the Periodic Table: Use a periodic table with clear annotations of atomic radius, ionization energy, electronegativity, and electron affinity trends.
-
Practice with Examples: Work through practice problems that require applying periodic trends to predict chemical behavior.
-
Connect Trends to Atomic Structure: Understand how the arrangement of electrons and the nuclear charge influence the properties of elements.
-
Develop Mnemonics: Create your own mnemonics or use existing ones to remember the trends and their exceptions.
Conclusion:
Periodic trends are essential concepts in chemistry, providing a framework for understanding and predicting the behavior of elements. By understanding the systematic changes in atomic radius, ionization energy, electron affinity, and electronegativity, we can gain insights into the reactivity, bonding properties, and applications of elements. Mastering periodic trends is fundamental for success in chemistry and related fields.
.PNG)
/periodictrendstable-5c4a46614cedfd000187c5db.jpg)


Closure
Thus, we hope this article has provided valuable insights into Understanding Periodic Trends: A Comprehensive Guide. We hope you find this article informative and beneficial. See you in our next article!