Understanding the Dynamics of Vapor Pressure: A Look Towards 2025
Understanding the Dynamics of Vapor Pressure: A Look Towards 2025
Introduction
In this auspicious occasion, we are delighted to delve into the intriguing topic related to Understanding the Dynamics of Vapor Pressure: A Look Towards 2025. Let’s weave interesting information and offer fresh perspectives to the readers.
Table of Content
Understanding the Dynamics of Vapor Pressure: A Look Towards 2025
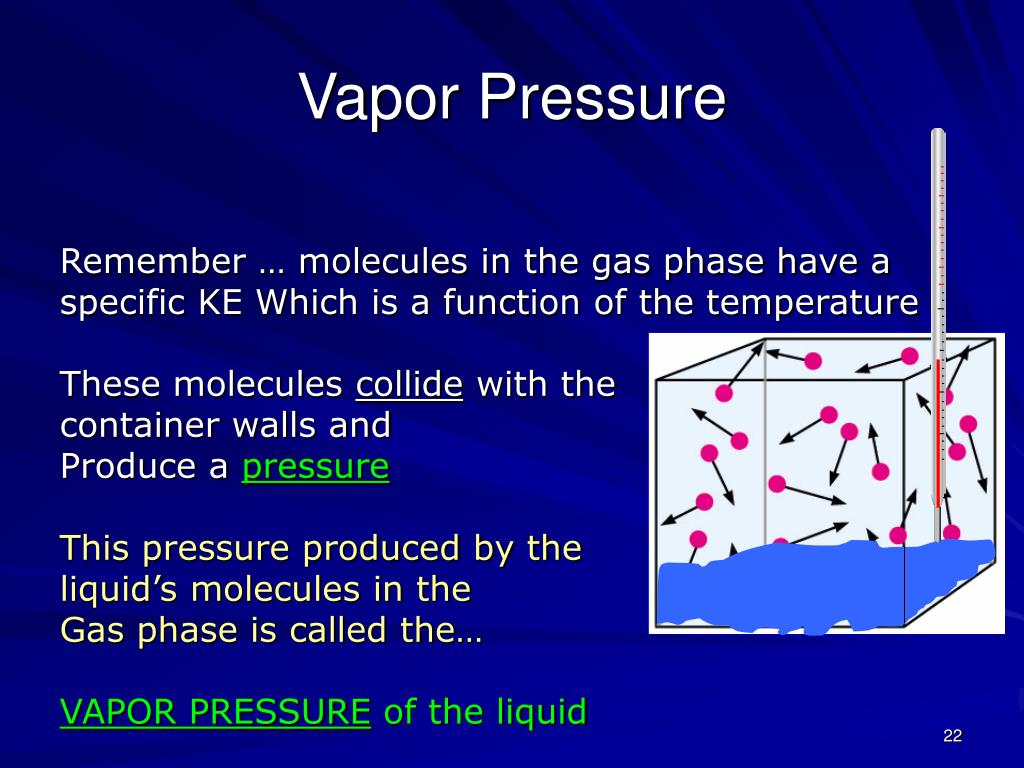
Vapor pressure, a fundamental thermodynamic property, governs the tendency of a liquid or solid to transition into a gaseous state. This seemingly simple concept holds profound implications across various scientific and industrial domains, impacting everything from climate modeling to the design of advanced materials.
As we approach 2025, understanding the trends in vapor pressure becomes increasingly crucial. The ever-evolving landscape of technology, coupled with the pressing need for sustainable solutions, necessitates a deeper understanding of how vapor pressure influences various aspects of our world.
Delving into the Essence of Vapor Pressure
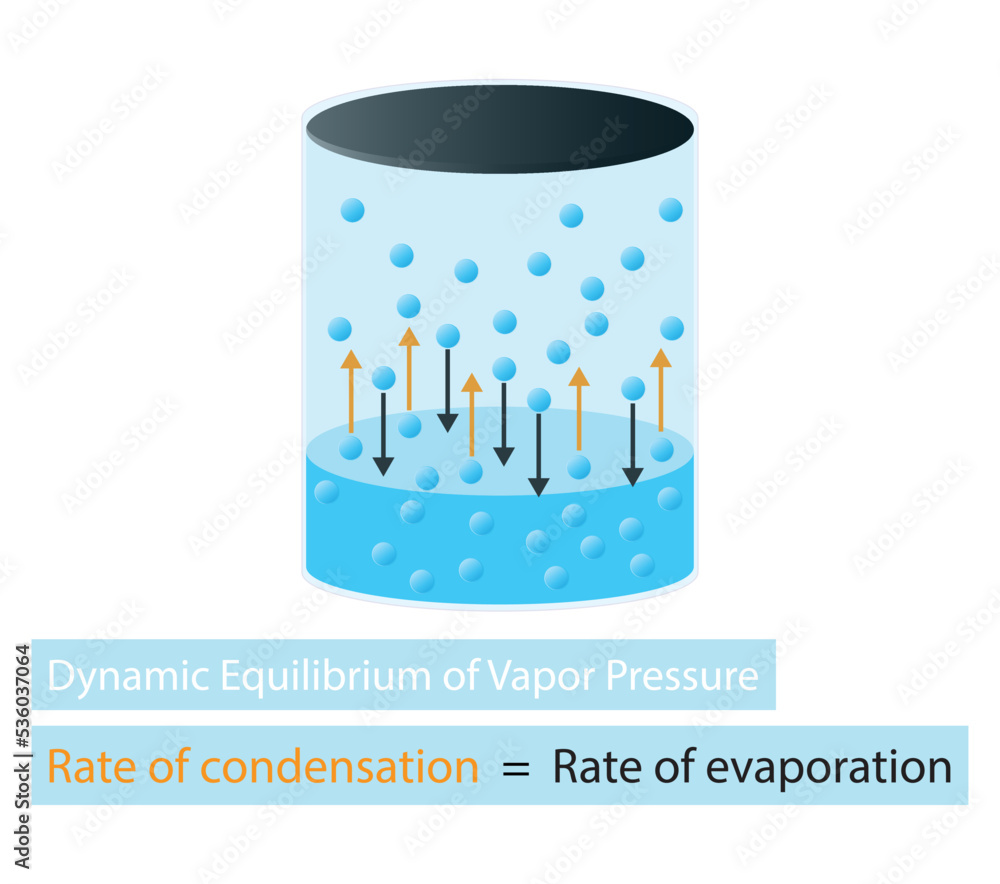
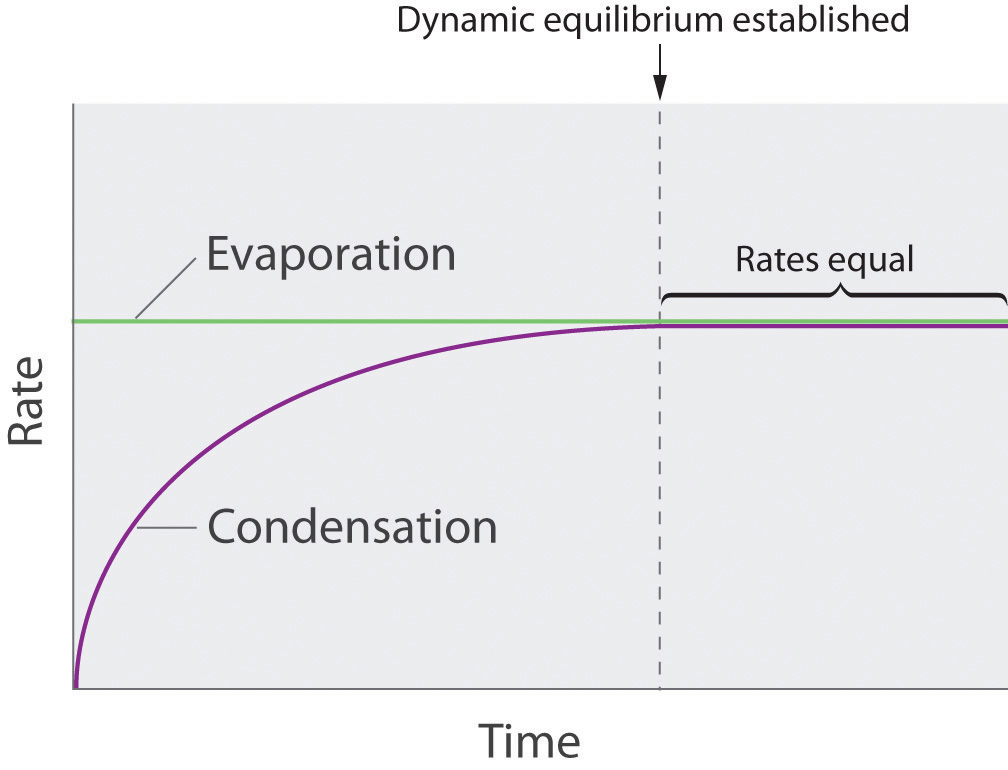
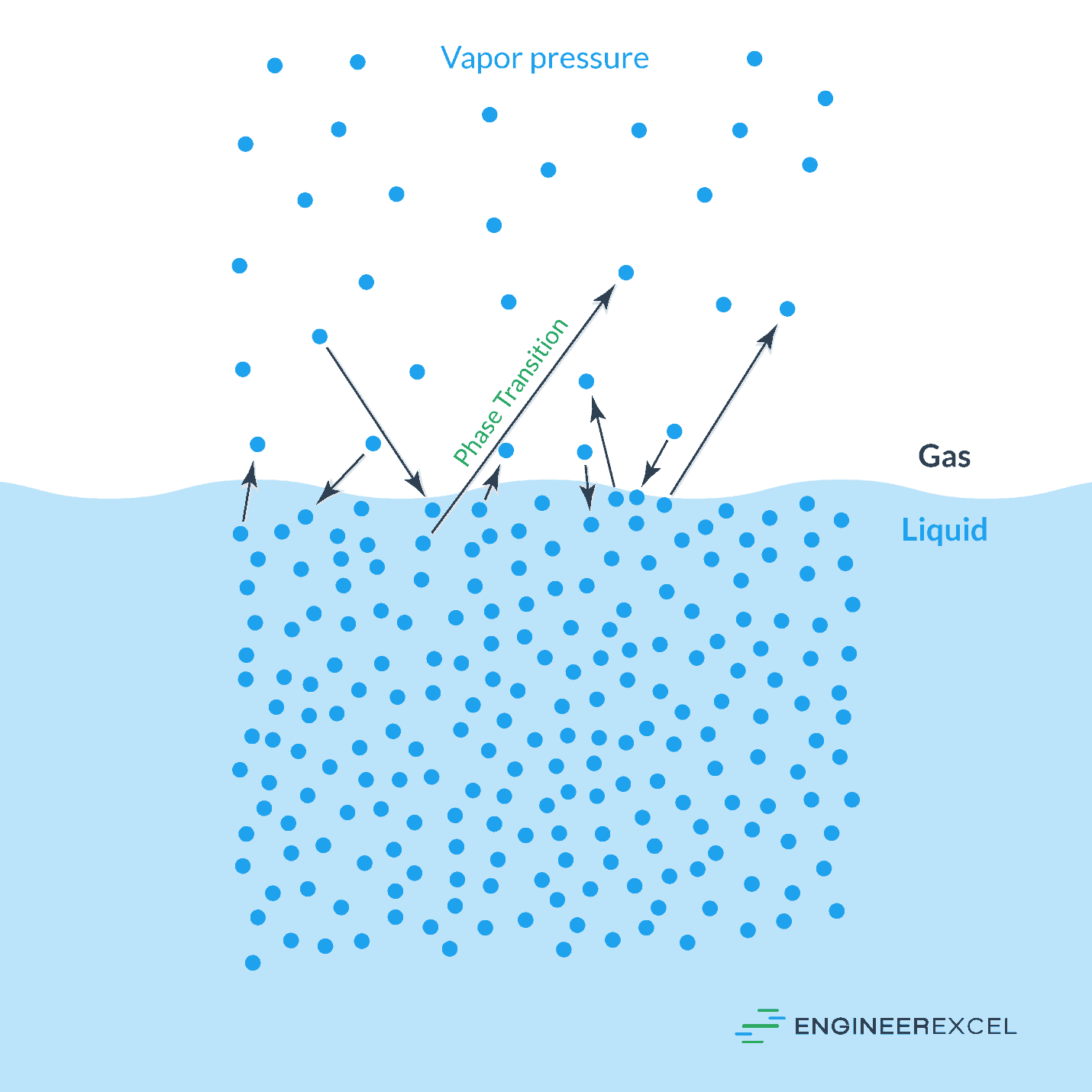
Vapor pressure is the pressure exerted by a vapor in thermodynamic equilibrium with its condensed phases (liquid or solid) at a given temperature. It represents the propensity of molecules to escape from the liquid or solid phase and enter the gas phase. Several factors influence vapor pressure, including:
- Temperature: As temperature rises, the kinetic energy of molecules increases, leading to a higher rate of vaporization and thus, a higher vapor pressure.
- Intermolecular Forces: Stronger intermolecular forces between molecules in a liquid or solid phase result in lower vapor pressure, as more energy is required to overcome these forces and enter the gas phase.
- Molecular Weight: Heavier molecules generally have lower vapor pressures, as they possess lower kinetic energy at a given temperature.
- Surface Area: A larger surface area exposed to the atmosphere allows for a greater rate of vaporization, leading to a higher vapor pressure.
The Significance of Vapor Pressure Trends in 2025
1. Climate Change and Atmospheric Modeling:
Vapor pressure plays a pivotal role in understanding and predicting climate change. The vapor pressure of water, a key greenhouse gas, directly influences the Earth’s energy balance and atmospheric humidity. As global temperatures rise, the vapor pressure of water increases, leading to more water vapor in the atmosphere, which further amplifies the greenhouse effect. Accurate climate models rely on precise calculations of vapor pressure, enabling scientists to predict future climate scenarios and develop mitigation strategies.
2. Advanced Materials Design:
Vapor pressure is a crucial factor in the design and development of advanced materials. For instance, in the field of pharmaceuticals, the vapor pressure of active ingredients influences their stability, shelf life, and delivery methods. Understanding vapor pressure trends allows scientists to optimize drug formulations and create more effective and stable medications.
3. Energy Efficiency and Sustainability:
Vapor pressure is central to energy efficiency and sustainability initiatives. For example, in refrigeration systems, the vapor pressure of refrigerants determines their performance and efficiency. The development of low-global-warming-potential (GWP) refrigerants with optimal vapor pressure characteristics is crucial for reducing environmental impact and improving energy efficiency.
4. Industrial Processes and Manufacturing:
Vapor pressure impacts various industrial processes, including distillation, evaporation, and drying. Optimizing vapor pressure conditions can enhance production efficiency, reduce energy consumption, and minimize waste generation. For instance, in the chemical industry, understanding vapor pressure trends allows engineers to design more efficient distillation columns for separating different components.
5. Environmental Monitoring and Pollution Control:
Vapor pressure is a critical factor in environmental monitoring and pollution control. The vapor pressure of volatile organic compounds (VOCs) influences their atmospheric concentrations and potential for air pollution. By monitoring vapor pressure trends, environmental agencies can assess air quality, identify pollution sources, and implement effective control measures.
Related Searches:
1. Vapor Pressure of Water:
The vapor pressure of water is a crucial parameter in various scientific and industrial applications. Understanding its temperature dependence is essential for accurate climate modeling, humidity control, and design of water-based systems.
2. Antoine Equation:
The Antoine equation is a widely used empirical formula that relates the vapor pressure of a pure substance to its temperature. This equation provides a practical tool for estimating vapor pressure values at different temperatures, crucial for various engineering and scientific applications.
3. Vapor Pressure Deficit:
Vapor pressure deficit (VPD) is the difference between the saturation vapor pressure and the actual vapor pressure in the air. VPD is a key indicator of atmospheric dryness and plays a significant role in plant physiology, water management, and agricultural productivity.
4. Clausius-Clapeyron Equation:
The Clausius-Clapeyron equation is a thermodynamic equation that relates the vapor pressure of a substance to its temperature and enthalpy of vaporization. This equation provides a theoretical framework for understanding the relationship between vapor pressure and temperature, crucial for predicting vapor pressure changes under different conditions.
5. Vapor Pressure Lowering:
Vapor pressure lowering is a colligative property that describes the decrease in vapor pressure of a solvent when a non-volatile solute is dissolved in it. This phenomenon is essential for understanding the behavior of solutions and is widely used in various applications, such as freezing point depression and boiling point elevation.
6. Raoult’s Law:
Raoult’s law states that the partial vapor pressure of a component in an ideal solution is equal to the vapor pressure of the pure component multiplied by its mole fraction in the solution. This law provides a fundamental framework for understanding the vapor pressure behavior of mixtures and is widely used in chemical engineering and process design.
7. Vapor Pressure Measurement:
Accurate measurement of vapor pressure is crucial for various scientific and industrial applications. Several techniques are employed for vapor pressure measurement, including static methods, dynamic methods, and effusion methods. The choice of method depends on the specific requirements of the application and the characteristics of the substance being measured.
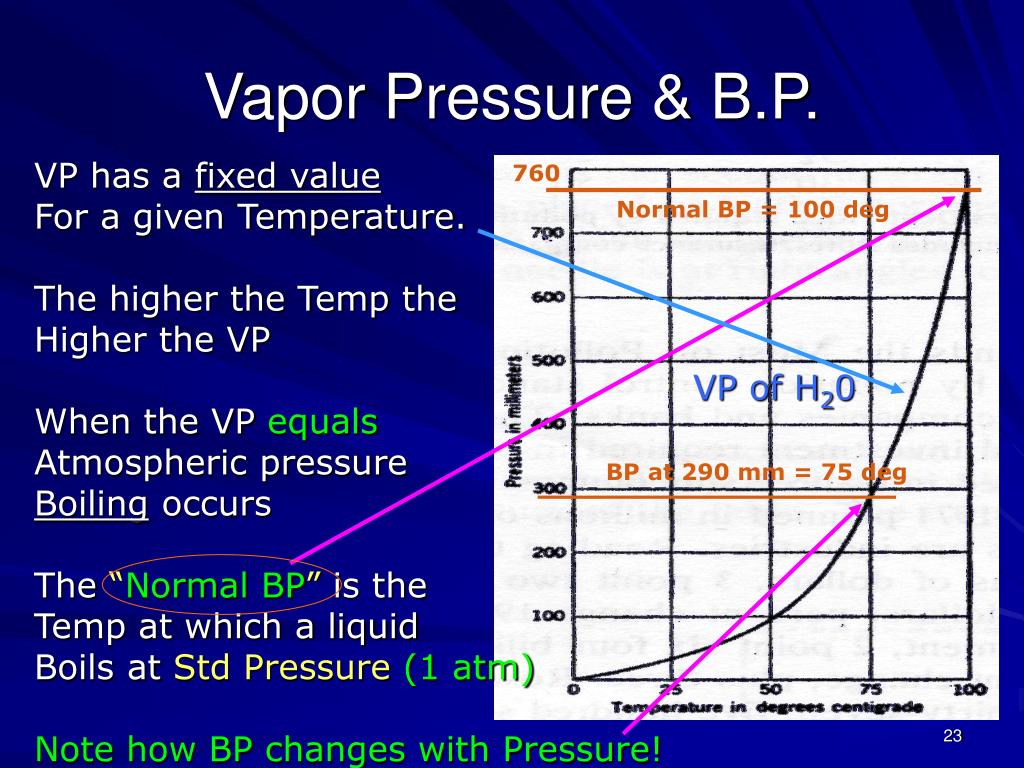
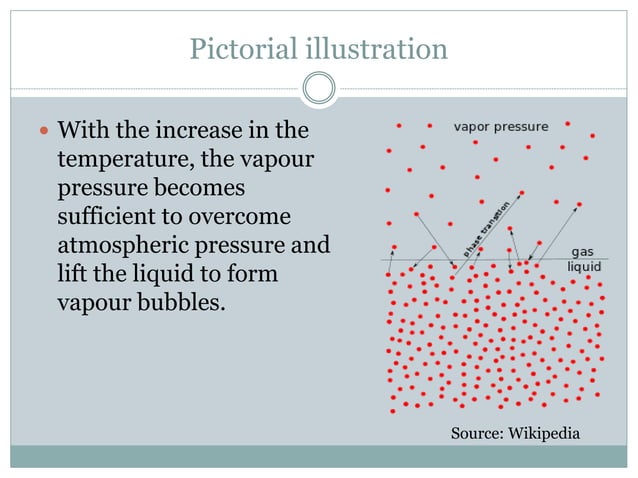
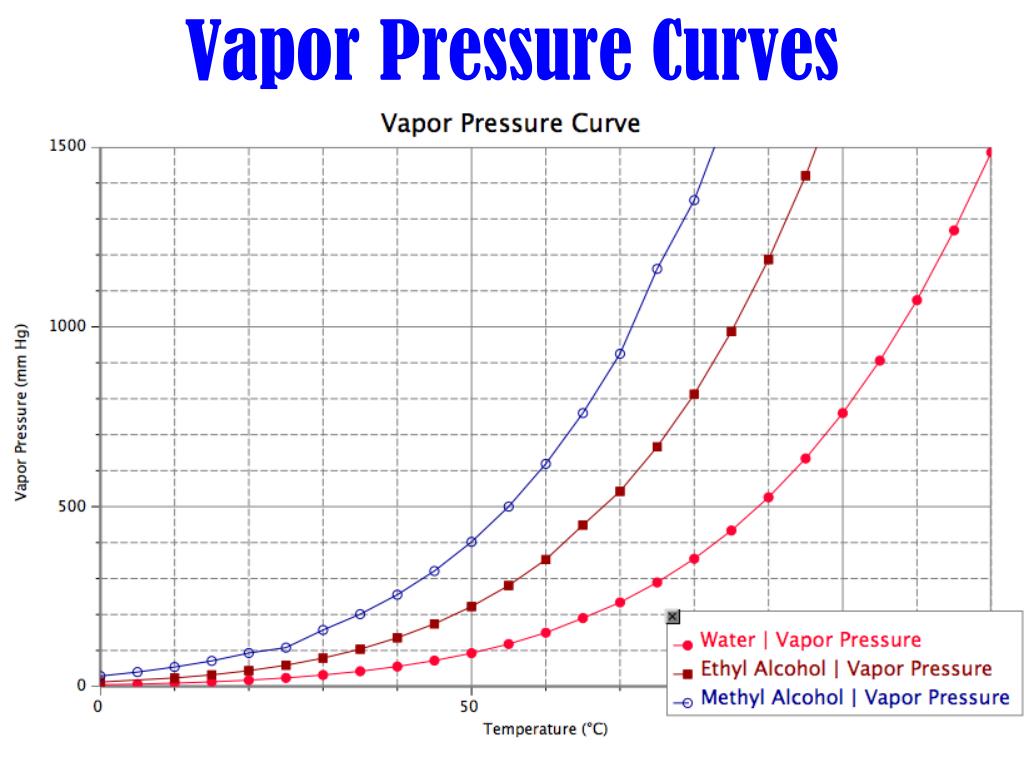
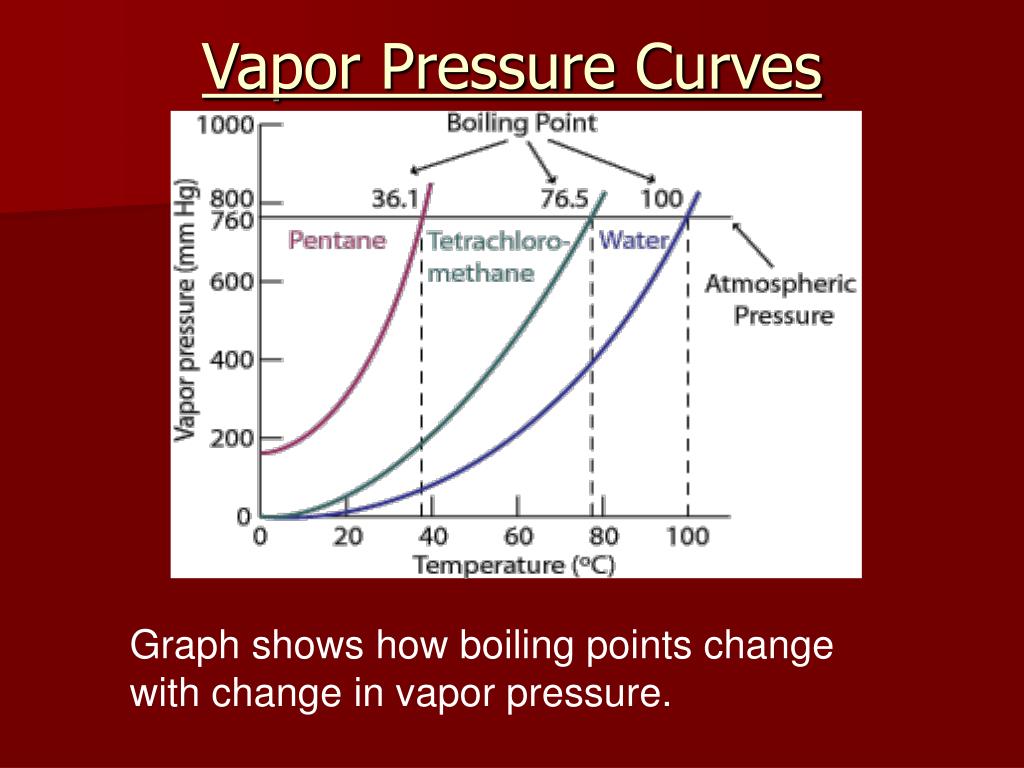
8. Vapor Pressure and Boiling Point:
The boiling point of a liquid is the temperature at which its vapor pressure equals the surrounding atmospheric pressure. Understanding the relationship between vapor pressure and boiling point is essential for various applications, including distillation, evaporation, and process design.
FAQs
1. How does vapor pressure relate to humidity?
Humidity refers to the amount of water vapor present in the air. The vapor pressure of water directly influences the humidity level. As the temperature increases, the vapor pressure of water also increases, leading to higher humidity.
2. What is the significance of vapor pressure in climate change?
Water vapor is a potent greenhouse gas, and its vapor pressure plays a crucial role in amplifying the greenhouse effect. As global temperatures rise, the vapor pressure of water increases, leading to more water vapor in the atmosphere, which further enhances the warming effect.
3. How does vapor pressure affect the boiling point of a liquid?
The boiling point of a liquid is the temperature at which its vapor pressure equals the surrounding atmospheric pressure. A higher vapor pressure at a given temperature indicates a lower boiling point, meaning the liquid will boil at a lower temperature.
4. What are some practical applications of vapor pressure knowledge?
Vapor pressure knowledge is crucial in various fields, including climate modeling, materials design, industrial processes, and environmental monitoring. It helps optimize drug formulations, design efficient refrigeration systems, control air pollution, and predict climate change scenarios.
5. What are the factors that influence vapor pressure?
Vapor pressure is influenced by several factors, including temperature, intermolecular forces, molecular weight, and surface area. As temperature rises, vapor pressure increases. Stronger intermolecular forces lead to lower vapor pressure. Heavier molecules generally have lower vapor pressures, while a larger surface area results in higher vapor pressure.
Tips for Understanding Vapor Pressure Trends
- Visualize the concept: Imagine molecules escaping from a liquid or solid surface and entering the gas phase. This visualization helps understand the fundamental principle of vapor pressure.
- Relate vapor pressure to temperature: Remember that vapor pressure increases with temperature. This relationship is key to understanding various applications of vapor pressure.
- Consider intermolecular forces: Stronger intermolecular forces in a liquid or solid phase lead to lower vapor pressure. This concept helps explain why some substances have higher vapor pressures than others.
- Apply vapor pressure knowledge to real-world scenarios: Think about how vapor pressure influences climate change, materials design, industrial processes, and environmental monitoring.
Conclusion
Vapor pressure is a fundamental thermodynamic property with far-reaching implications across various scientific and industrial domains. Understanding the trends in vapor pressure is crucial for addressing pressing challenges related to climate change, materials design, energy efficiency, and environmental monitoring. As we move towards 2025 and beyond, a deeper understanding of vapor pressure dynamics will be essential for developing innovative solutions and ensuring a sustainable future.

Closure
Thus, we hope this article has provided valuable insights into Understanding the Dynamics of Vapor Pressure: A Look Towards 2025. We hope you find this article informative and beneficial. See you in our next article!