Unveiling the Secrets of the Periodic Table: A Guide to Exploring Periodic Trends
Unveiling the Secrets of the Periodic Table: A Guide to Exploring Periodic Trends
Introduction
In this auspicious occasion, we are delighted to delve into the intriguing topic related to Unveiling the Secrets of the Periodic Table: A Guide to Exploring Periodic Trends. Let’s weave interesting information and offer fresh perspectives to the readers.
Table of Content
Unveiling the Secrets of the Periodic Table: A Guide to Exploring Periodic Trends

The periodic table, a cornerstone of chemistry, is a remarkable tool that organizes the elements based on their properties. Understanding the periodic trends within this table is crucial for comprehending the behavior of elements and predicting their reactivity.
Student Exploration: Periodic Trends Gizmo is an invaluable resource for students seeking to grasp these fundamental concepts. This interactive tool provides a dynamic and engaging platform for exploring the periodic trends, fostering a deeper understanding of the underlying principles.
Navigating the Periodic Table: A Journey Through Trends
The periodic table is organized into rows (periods) and columns (groups). Elements within the same group share similar chemical properties due to having the same number of valence electrons, the electrons in the outermost shell.
Student Exploration: Periodic Trends Gizmo allows students to visualize and analyze these trends by manipulating variables such as atomic radius, ionization energy, electronegativity, and electron affinity.
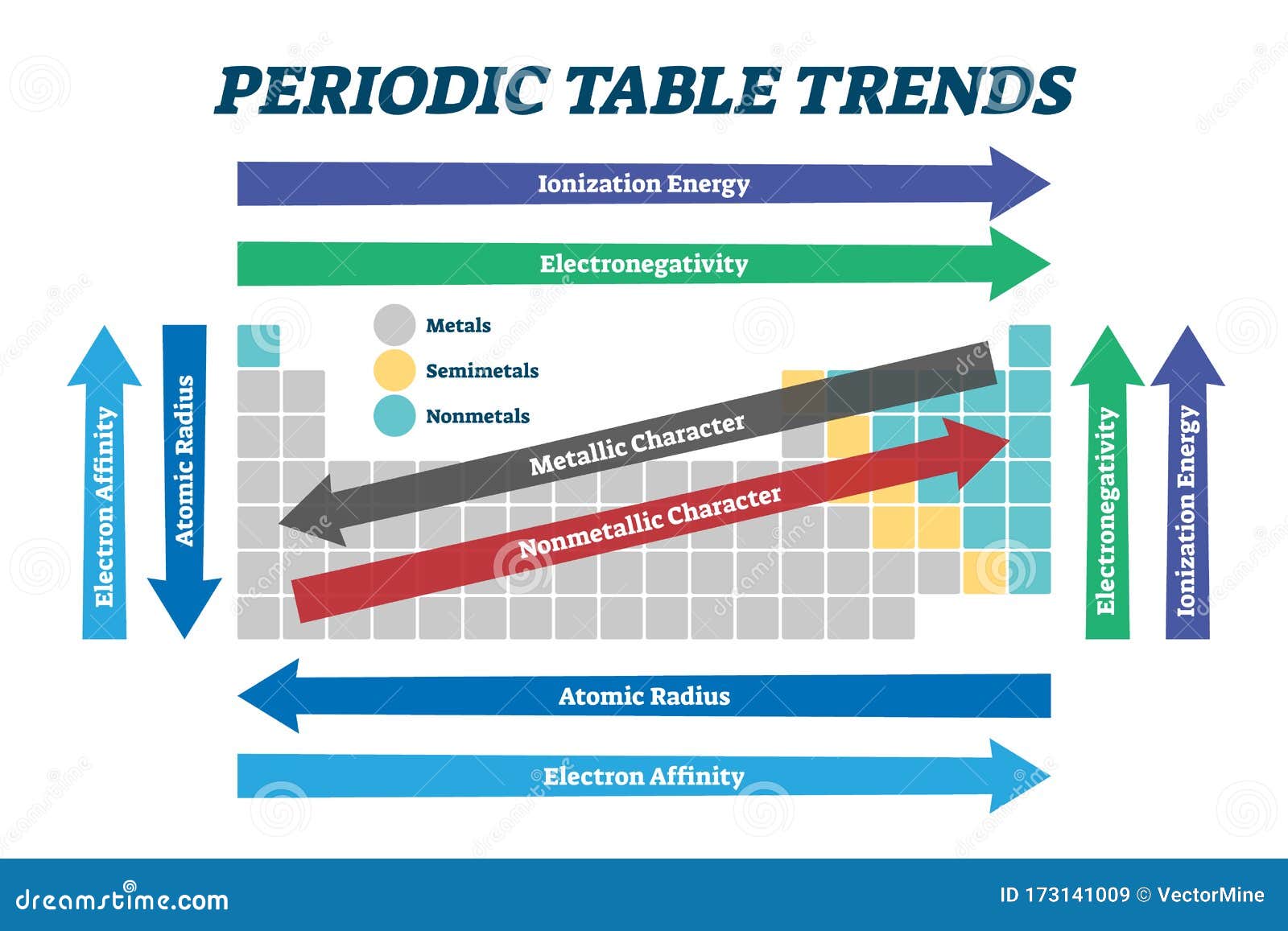
1. Atomic Radius: This trend describes the size of an atom, measured as the distance between the nucleus and the outermost electron shell. As you move down a group, the atomic radius increases due to the addition of electron shells. Conversely, moving across a period from left to right, the atomic radius decreases as the effective nuclear charge (the attraction between the nucleus and the outermost electrons) increases.
2. Ionization Energy: This refers to the minimum energy required to remove an electron from a gaseous atom. Ionization energy generally increases as you move across a period due to the stronger attraction between the nucleus and electrons. Moving down a group, ionization energy decreases as the outermost electron is farther from the nucleus and less tightly bound.
3. Electronegativity: This property measures an atom’s ability to attract electrons in a chemical bond. Electronegativity generally increases across a period as the effective nuclear charge increases, making the atom more attractive to electrons. It decreases down a group as the outermost electrons are farther from the nucleus and less tightly bound.
4. Electron Affinity: This refers to the change in energy when an electron is added to a neutral atom in the gaseous state. Electron affinity generally increases across a period due to the increased attraction between the nucleus and electrons. However, it can exhibit some irregularity as you move down a group.
Benefits of Using Student Exploration: Periodic Trends Gizmo
Student Exploration: Periodic Trends Gizmo offers numerous benefits for students learning about periodic trends:
- Interactive Learning: The gizmo provides a dynamic and engaging platform for exploring the concepts. Students can manipulate variables and observe the resulting changes in the properties of elements.
- Visual Representation: The gizmo utilizes visual representations of atoms and their properties, making it easier for students to grasp abstract concepts.
- Self-Paced Learning: Students can work at their own pace, exploring the concepts in depth or focusing on specific areas of interest.
- Reinforcement of Concepts: The gizmo provides opportunities for students to test their understanding through interactive exercises and quizzes.
- Real-World Applications: The gizmo connects the concepts of periodic trends to real-world applications, helping students understand the relevance of these principles.
Related Searches: Exploring the Wider Context
1. Periodic Trends Worksheet Answers: Numerous online resources provide worksheets and answer keys for students to practice their understanding of periodic trends. These resources can be used alongside Student Exploration: Periodic Trends Gizmo to reinforce learning.
2. Periodic Trends Quizlet: Quizlet offers interactive flashcards and study sets specifically designed to help students learn and memorize periodic trends. These resources provide a convenient way for students to test their knowledge and identify areas that need further review.
3. Periodic Trends Chart: A periodic trends chart is an essential tool for visualizing and understanding these trends. Many websites and textbooks offer downloadable periodic trends charts that can be used for reference and study.
4. Periodic Trends Practice Problems: Practice problems are crucial for applying the concepts of periodic trends to real-world scenarios. Numerous online resources and textbooks provide practice problems with detailed solutions.
5. Periodic Trends and Chemical Bonding: Understanding periodic trends is essential for comprehending the formation of chemical bonds. The relationship between periodic trends and chemical bonding can be explored through additional resources and activities.
6. Periodic Trends and Reactivity: The reactivity of elements is directly linked to their position on the periodic table and the corresponding periodic trends. Exploring the relationship between periodic trends and reactivity can enhance understanding of chemical reactions.
7. Periodic Trends and Atomic Structure: Understanding the atomic structure of elements is fundamental to grasping periodic trends. Exploring the relationship between atomic structure and periodic trends provides a deeper understanding of the underlying principles.
8. Periodic Trends and Group Trends: Understanding the trends within specific groups of elements is essential for predicting the behavior of elements in those groups. Exploring the group trends within the periodic table can further enhance understanding of the periodic trends.
FAQs: Addressing Common Questions
Q1: What are the main periodic trends?
A: The main periodic trends include atomic radius, ionization energy, electronegativity, and electron affinity. These trends are based on the arrangement of electrons in atoms and their interactions with the nucleus.
Q2: How do periodic trends affect the reactivity of elements?
A: Elements with lower ionization energies and higher electronegativity tend to be more reactive. For example, elements in Group 1 (alkali metals) have low ionization energies and readily lose electrons, making them highly reactive.
Q3: What are some real-world applications of periodic trends?
A: Periodic trends have numerous real-world applications, including:
- Predicting the properties of new elements: Understanding periodic trends allows scientists to predict the properties of new elements even before they are synthesized.
- Developing new materials: Periodic trends help in designing new materials with specific properties, such as high conductivity or strength.
- Understanding chemical reactions: Periodic trends provide insights into the reactivity of elements and the types of chemical bonds they form.
Tips for Mastering Periodic Trends
- Visualize the Trends: Use periodic trends charts and interactive tools like Student Exploration: Periodic Trends Gizmo to visualize the trends and their relationships.
- Practice, Practice, Practice: Solve practice problems and quizzes to test your understanding and identify areas that need further review.
- Connect the Concepts: Understand the relationship between periodic trends, atomic structure, chemical bonding, and reactivity.
- Explore Real-World Examples: Look for real-world examples of how periodic trends are applied in different fields, such as medicine, technology, and industry.
Conclusion: Embracing the Power of Periodic Trends
Student Exploration: Periodic Trends Gizmo provides a valuable platform for students to explore the fascinating world of periodic trends. By understanding these trends, students gain a deeper understanding of the behavior of elements, their reactivity, and their role in shaping the world around us.
The periodic table is a testament to the order and beauty of the natural world. By embracing the power of periodic trends, students can unlock a deeper understanding of the fundamental principles governing the behavior of matter and its interactions.






![[PDF] Exploring the Elements: A Complete Guide to the Periodic Table](https://www.yumpu.com/en/image/facebook/67675648.jpg)
Closure
Thus, we hope this article has provided valuable insights into Unveiling the Secrets of the Periodic Table: A Guide to Exploring Periodic Trends. We appreciate your attention to our article. See you in our next article!